Why is Ice Solid & Water is Liquid?

We all know that water is essential for the survival of all the living beings, whether it is trees, human beings or animals. We also need water for our day to day household activities such as washing clothes and dishes, cooking food, bathing, watering the plants and the list does not end here. Most importantly we need water to drink and fulfill the requirements of the body and even avoid the situation of dehydration (a state where the body is in urgent need of any liquid as being made of 70% water, it dries up without sufficient water intake and can even cause death).
Rather than talking about the human body, the water constitutes 71% of the whole planet Earth in the form of lakes, rivers, oceans, seas and other water bodies.
Water is a transparent, tasteless, colorless, odorless and reaction-less substance which has no specified shape or state, which are all not just its definition but are its properties too. Water is found in all three states present in the mother nature, which is, existence as ice and snow in the solid state, water as fluid or liquid state and water vapor or steam in gaseous form.
After all, we all have sometimes visited the snowy mountains and hill stations or glaciers with ice, or been near any river bank and have experienced rainfall at least once, which gives us clear practical knowledge and picture of all the three states existing in nature.
All the three states are very important for the survival of all the living beings on the planet, Earth and even the rejuvenation of soil. Without even a single state the water cycle would be incomplete. You must be wondering what Water Cycle is?
The water cycle is a process through which the water present in the water bodies such as ponds, lakes, Seas, rivers, and oceans evaporates into the vapor formation and starts rising up because of formation of steam when it reaches a height where the atmosphere is cool, they condense to form clouds. These clouds when getting heavy with more and more water clinging to it, and reaches its maximum capacity where it cannot hold of the weight of water droplets gathering anymore, the water is sent back to land in form of rainfall.
As we know that ice is the frozen solid state of water. Ice exists in nature in the form of glaciers, icebergs, snow (when any dust articles mix with it which gives it a bit of opaque whitish look rather than its crystal clear form) and etc. It even works as a reserve of fresh water source present in nature, where it can melt to its liquid state.
State of any substance or matter is a play of molecular structures.
We know that the chemical formula for water is H20. which means it is formed by combining two hydrogen molecules with one Oxygen to form water.
Do you know what molecule is?
Molecules and Atoms are the smallest parts, the building blocks of any matter which combine to form a substance. When these molecules are very tightly and compactly packed it is known as the solid state. It has no space between its molecules, opaque, tangible, brittle being some of the properties of the solid state. When the molecules are less in number or are loosely packed as compared to the solid state, it is known to be the liquid state which enables the molecules to have a better flexibility and move around giving liquids their property of free-flowing and having no particular shape. Similarly, when the molecules are completely loose and very very freely packed almost on the verge of separating, they are very flowy and light which gives them the consistency of gases.
As we know that the water exists in all three forms solids liquids and gases.
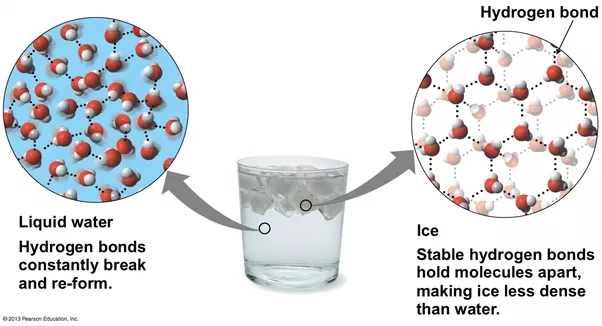
It is not only the molecule built which differentiate state but also it is affected by the temperature of the surroundings whatever is usually found in liquid state which is a fluid form when at room temperature. At the room temperature, the molecules are comparatively loose to give it a fluid formation. Every substance has a boiling and melting point as well as a freezing point. Water condenses to form ice and when it reaches its freezing point and when it reaches its boiling point is starts evaporating into steam or vapors which is the gaseous form.
The freezing point of water is zero degree Celsius. Water’s molecules dense with the temperature and get tightly and compactly packed, when water reaches the areas (hill stations) where the temperature of surroundings is zero or in negative, and hence the water here is found in its condensed, solid state, that is ice.
(When the water reaches above 100 degree Celsius of temperature due to heat present in the environment, it evaporates too, to form vapors.)
Hence this is why ice is solid and water is liquid.





Responses